- SolutionsOne platform. All your EHS & ESG needs.Designed to configure and scale, build your EHS & sustainability solution set within a single award-winning platform to empower everyone at every level across your organization to make responsible business decisions.Product CollectionsProtect your global workforce >>Enable better preventions >>Ensure accurate sustainability >>Transform compliance >>Better operational value >>Actionable performance insights >>Featured SolutionsProduct HighlightCority's Hygiene Essentials Solution
Wins Prestigious 2023 OH&S
Industrial Hygiene Award
- Solutions
- CorityOneTM
Achieve higher levels of operational and sustainable performance with our comprehensive SaaS platform.Learn More
Health CloudProtect your global workforce from risk and optimize compliance for improved worker health.
Safety CloudEnable better prediction and preventions to keep your workforce and workplace safe.
Sustainability CloudEnsure accurate and auditable sustainability and ESG data to create a greener future.
Environmental CloudTransform compliance and drive continuous improvement to meet your environmental goals.
Quality CloudReduce complexity and increase quality within your supply chain for better operational value.
Analytics CloudHarness your data and transform it into actionable insights for better organizational performance.
- Featured Solutions
Occupational Health Industrial Hygiene Investor ESG Management Audits & Inspections Air Emissions Management Customer Experience
- Product Highlight
Sustainability Performance Management Software - Helping you achieve your sustainability goals.
For sustainability, ESG, and EHS teams who need to streamline data collection, monitor sustainability impacts, meet reporting requirements, and increase stakeholder visibility. Learn More
- Who We Serve
Featured Category
Private Market Investing
Designed for private market investors, Cority's market-leading solutions empower responsible investment decisions at every step of the ESG journey.
- Who We Serve
- Resources
- Resource Center A valuable source for your continued learning, latest updates, and cross-industry thinking.Learn More
- Featured eBookPreventing SIFs with Digitization: Reduce Serious Injuries and Fatalities with Technology
- Featured BlogHow Cority’s EHS Product Roadmap Excels at Meeting Marketplace Needs
- Featured WebinarEfficiency unleashed: Transforming flu shot clinics with software technology
- Featured Resource
Cority Recognized as a Leading Provider for ESG Reporting and Data Management Software
Learn More

- About Us
Request a Demo Featured AwardFujitsu and Cority Win Climate Innovation Award for Scope 3 & Supply Chain Sustainability Collaboration
Featured AwardFujitsu and Cority Win Climate Innovation Award for Scope 3 & Supply Chain Sustainability Collaboration Community NewsNew Digital Badge Program Elevates Cority Software Users as Platform Experts
Community NewsNew Digital Badge Program Elevates Cority Software Users as Platform Experts
- Featured News
Cority Wins Exposure Assessment & Risk Management Categories in Prestigious 2024 OH&S Industrial Hygiene Award
Learn More


- Solutions
- Solutions
- CorityOneTM
- Health Cloud
- Safety Cloud
- Sustainability Cloud
- Environmental Cloud
- Quality Cloud
- Analytics Cloud
- Featured Solutions
- Occupational Health
- Industrial Hygiene
- Investor ESG Management
- Audits & Inspections
- Air Emissions Management
- Customer Experience
- Sustainability Performance Management Software – Helping you achieve your sustainability goals.
For sustainability, ESG, and EHS teams who need to streamline data collection, monitor sustainability impacts, meet reporting requirements, and increase stakeholder visibility.
- Who We Serve
- Who We Serve
- Resources
- About Us
- CorityOneTM
Achieve higher levels of operational and sustainable performance with our comprehensive SaaS platform.Learn More
Elevate Your Business with Quality Management Software
Cority’s Quality Cloud lets you leave the paper behind and benefit from powerful software that streamlines quality management.


Better quality management matters
Your quality management processes should be as unique as your workforce – and software solutions that can flex, grow, and adapt to the changing needs of your programs are vital.
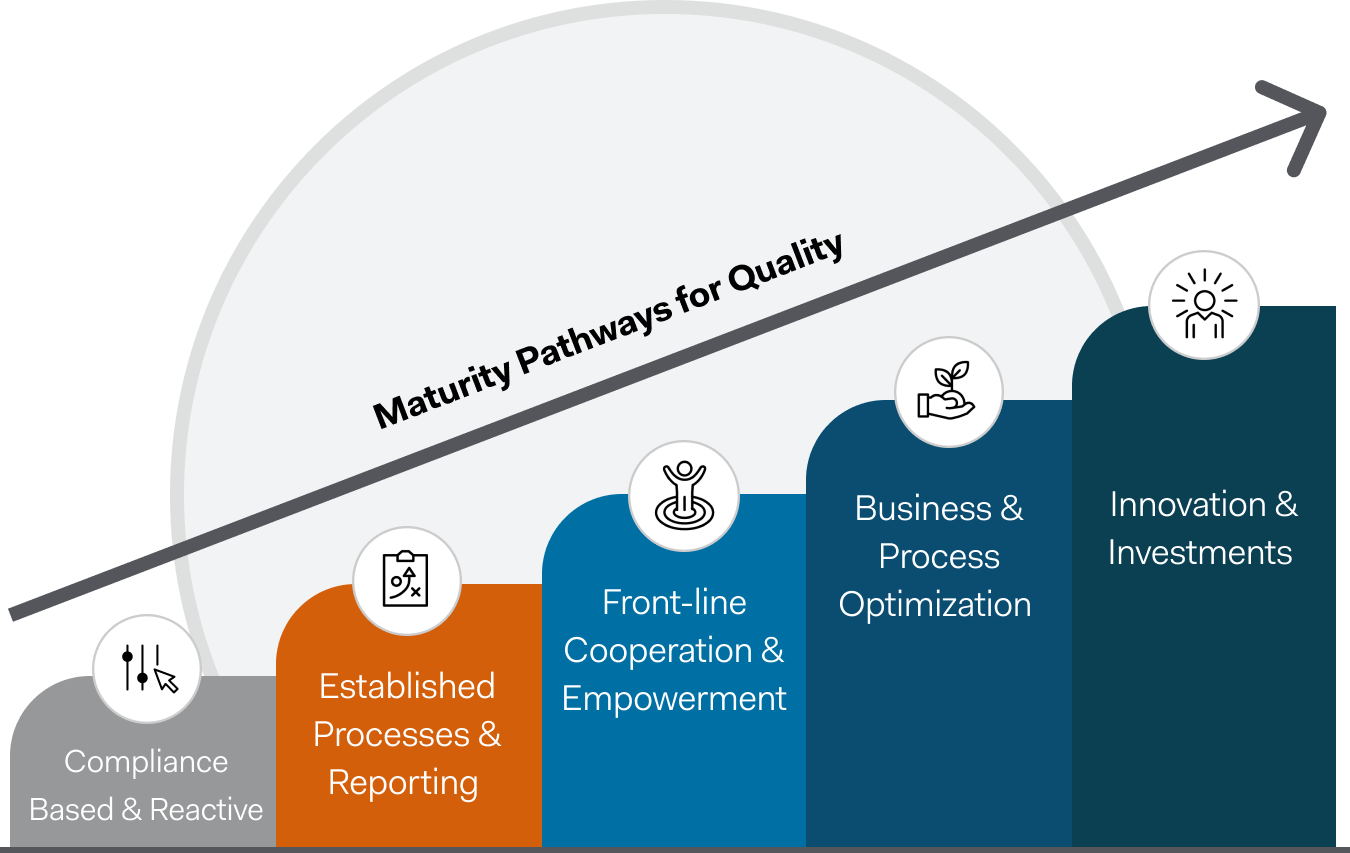
Market-leading safety management software

Voter’s Choice
Canadian Occupational Safety’s 5-Star OHS Software and Technology – Voter’s Choice, Quality Essentials

Leader Award
Verdantix Leader Award 2023 – Leader in Process Safety Management Software
-
 Testimonial
Testimonial“Before tracking supplier communications in Cority, our response time for suppliers getting back to us was about five days. Since we let our suppliers know that we are now tracking communications in Cority, we have seen their response time drop down to approximately three from the original five days.”
Nicholas Sondelski, Information Systems Administrator, Pointe Precision
-
 Testimonial
Testimonial“We’ve been able to use quality as a weapon. For example, when a vendor brings up higher pricing, we talk quality and essentially, we never forget a thing. The data is always there, instantaneously available at our fingertips to support our position.”
Doug Jones, Quality Assurance Manager, Parker Hannifin
-
 Testimonial
Testimonial“Cority has allowed us to identify our problem areas, focus on them, and build prevention programs to help drive prevention in those areas.”
Pierre Perrier, National Manager of Transportation Safety, Purolator
Modular, purpose-built quality solutions to meet your needs
Solution
Features
Document Automation for EHS & Quality
Provides secure, fast, and efficient documentation access to all stakeholders locally or globally with minimum administrative overhead and maximum control.
Efficiently assure compliance
Purpose-built to digitize paper-based processes associated with EHSQ documentation requirements. Take human error and information overload out of the compliance equation.
Full lifecycle management
Store and retrieve files from solutions spanning CorityOne™, maintain version control, schedule reviews, and monitor the status of files through an intuitive UI to the configurable approval process.
Plan For Change And Mitigate Risks
Automate the process to ensure changes are properly reviewed before they are implemented to avoid, minimize, and mitigate key environmental, health, and safety risks.
Maintain Regulatory Compliance Through Change
Gain insights and readily handle competing priorities, such as implementing the change on time and budget, of providing training to impacted employees.
Centralize And Standardize Change Management
Plan for change, assess risks and impacts, determine regulatory and policy obligations, administer the change, manage training, execute communications, and measure the success of change through a standard and automated process.
Centralized Control
Provide administrators with a central access point to view and manage their full audit or inspection programs, including completion status and history of the related findings and actions.
Streamlined Workflows
Schedule recurring or one-time activities and view them in a simple, intuitive calendar that allows users to see upcoming activities at a glance.
Empowered Mobile Workforce
myCority empowers your employees to complete audit and inspection tasks through an intuitive interface – enabling them to complete questionnaires, audit checklists, record findings, and stay on top of their assigned work.
Enhance your view of operational risk
Enhance the visibility and awareness of operational risk across the business to drive better decisions that help mitigate losses and support better operational performance with flexible, real-time access to risk data across the entire Cority risk management software platform.
Strengthen compliance
Improve organizational compliance to applicable risk management regulations, standards, and corporate policies. Mitigate the risk of loss, reduce exposure to regulatory citations and penalties, and protect your brand.
Drive engagement
Leverage purpose-built tools to help share risk information broadly across the workforce to support better risk control and drive greater employee participation in risk management actions and decisions.


Streamline Process
Automate and streamline shop floor processes, manage the entire nonconformance lifecycle, and support enterprise-wide continuous improvement initiatives as a stand-alone EQMS or integrated with EHS.
Increase Visibility
Gain actionable insight into performance, cost of quality and risk-related metrics, monitor suppliers, capture customer feedback, and ensure the highest product standards.
Supplier Performance
Transform the way you engage, collaborate, track, and improve all supplier related quality management activities in a secure and centralized system.
Prioritize quality management for operational excellence
Designed to protect our customer’s global workforce from operational risk and streamline, automate, and improve compliance for better worker performance.
Resources curated by quality management, process and software experts


Guidance from Cority goes beyond software. From blogs, to guides, to webinars, our team is dedicated to empowering better living and health program excellence
Our team of industry experts has created an extensive library of resources on topics, like the below, to help organizations stay in-the-know on evolving best-practices, industry news, and current trends related to quality program management.
-
Featured Guide
Unifying Quality and Safety Management with a Single Solution Provider

-
Featured Content
Voices of Cority – The Cost of Poor Quality with David Hartmann

-
Featured Blog
The Benefits of Management of Change Software

How can Quality Management change your world?
Request a DemoMenu
Safety Solutions
Stay Informed!
Sign up for EHS and ESG news and best practices. Get notified on upcoming webinars, exclusive events, and industry news.