- SolutionsOne platform. All your EHS & ESG needs.Designed to configure and scale, build your EHS & sustainability solution set within a single award-winning platform to empower everyone at every level across your organization to make responsible business decisions.Product CollectionsProtect your global workforce >>Enable better preventions >>Ensure accurate sustainability >>Transform compliance >>Better operational value >>Actionable performance insights >>Featured SolutionsProduct HighlightCority's Hygiene Essentials Solution
Wins Prestigious 2023 OH&S
Industrial Hygiene Award
- Solutions
- CorityOneTM
Achieve higher levels of operational and sustainable performance with our comprehensive SaaS platform.Learn More
Health CloudProtect your global workforce from risk and optimize compliance for improved worker health.
Safety CloudEnable better prediction and preventions to keep your workforce and workplace safe.
Sustainability CloudEnsure accurate and auditable sustainability and ESG data to create a greener future.
Environmental CloudTransform compliance and drive continuous improvement to meet your environmental goals.
Quality CloudReduce complexity and increase quality within your supply chain for better operational value.
Analytics CloudHarness your data and transform it into actionable insights for better organizational performance.
- Featured Solutions
Occupational Health Industrial Hygiene Investor ESG Management Audits & Inspections Air Emissions Management Customer Experience
- Product Highlight
Sustainability Performance Management Software - Helping you achieve your sustainability goals.
For sustainability, ESG, and EHS teams who need to streamline data collection, monitor sustainability impacts, meet reporting requirements, and increase stakeholder visibility. Learn More
- Who We Serve
Featured Category
Private Market Investing
Designed for private market investors, Cority's market-leading solutions empower responsible investment decisions at every step of the ESG journey.
- Who We Serve
- Resources
- Resource Center A valuable source for your continued learning, latest updates, and cross-industry thinking.Learn More
- Featured eBookPreventing SIFs with Digitization: Reduce Serious Injuries and Fatalities with Technology
- Featured BlogHow Cority’s EHS Product Roadmap Excels at Meeting Marketplace Needs
- Featured WebinarEfficiency unleashed: Transforming flu shot clinics with software technology
- Featured Resource
Cority Recognized as a Leading Provider for ESG Reporting and Data Management Software
Learn More

- About Us
Request a Demo Featured AwardFujitsu and Cority Win Climate Innovation Award for Scope 3 & Supply Chain Sustainability Collaboration
Featured AwardFujitsu and Cority Win Climate Innovation Award for Scope 3 & Supply Chain Sustainability Collaboration Community NewsNew Digital Badge Program Elevates Cority Software Users as Platform Experts
Community NewsNew Digital Badge Program Elevates Cority Software Users as Platform Experts
- Featured News
Cority Wins Exposure Assessment & Risk Management Categories in Prestigious 2024 OH&S Industrial Hygiene Award
Learn More


- Solutions
- Solutions
- CorityOneTM
- Health Cloud
- Safety Cloud
- Sustainability Cloud
- Environmental Cloud
- Quality Cloud
- Analytics Cloud
- Featured Solutions
- Occupational Health
- Industrial Hygiene
- Investor ESG Management
- Audits & Inspections
- Air Emissions Management
- Customer Experience
- Sustainability Performance Management Software – Helping you achieve your sustainability goals.
For sustainability, ESG, and EHS teams who need to streamline data collection, monitor sustainability impacts, meet reporting requirements, and increase stakeholder visibility.
- Who We Serve
- Who We Serve
- Resources
- About Us
- CorityOneTM
Achieve higher levels of operational and sustainable performance with our comprehensive SaaS platform.Learn More
Make better decisions at every level with integrated, scalable solutions for
Explore our EHS & Sustainability Clouds








One platform. All your EHS & ESG needs.
Now everyone at every level can gather and gain insight from across the organization with the most comprehensive, fully integrated EHS & Sustainability SaaS platform.
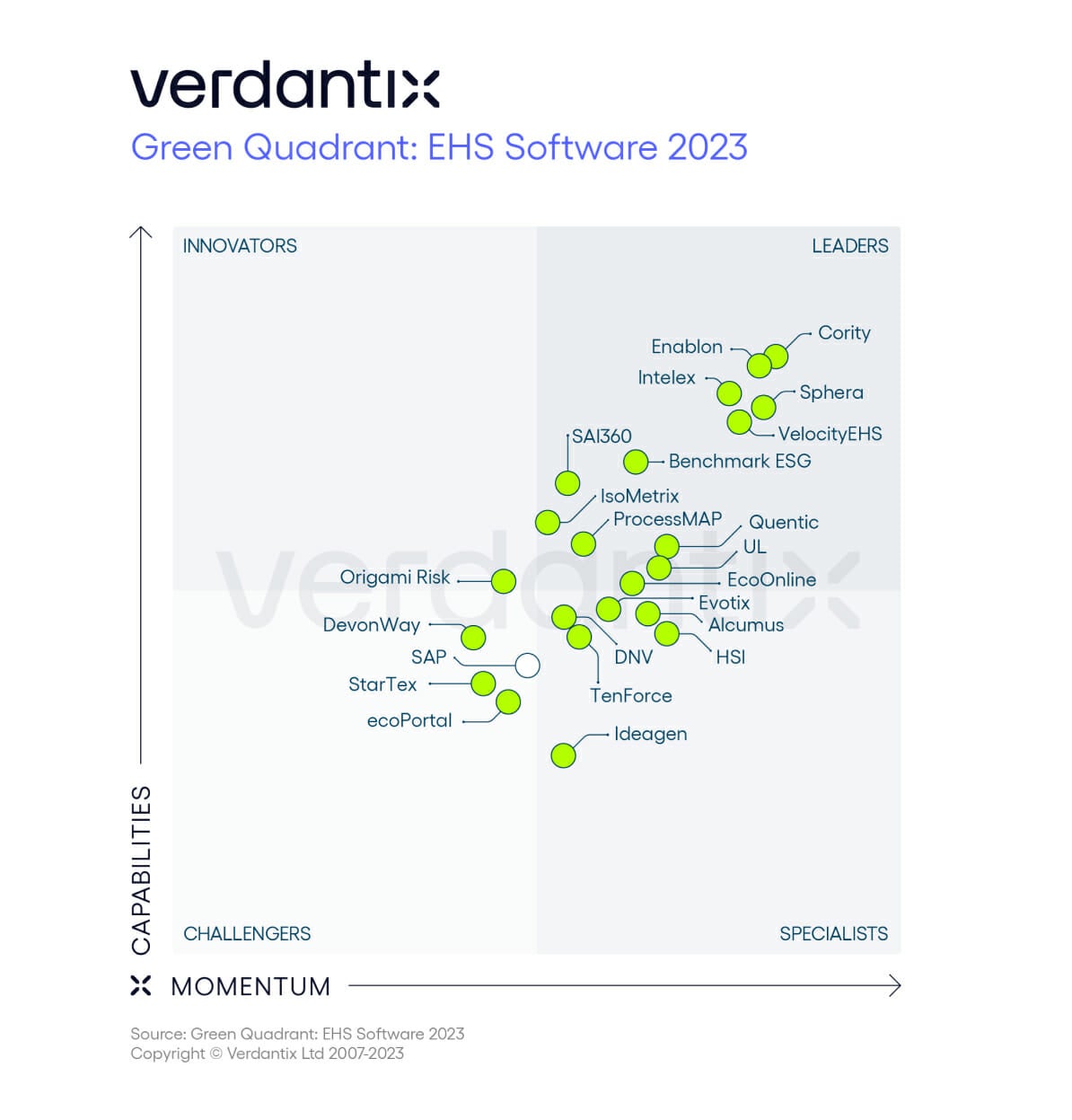
Known for EHS software leadership.
Consistently recognized for innovation.
2015

2017

2019

2021

2023

2015 – 2023

From investment screening to and due diligence to portfolio management, we’ve got solutions for every step of the ESG journey
From the field to the floor to the office, and everywhere in between — we got you.







Helping enterprises worldwide do better since 1989.
People-first solutions that drive better EHS & ESG performance.
Empower everyone to make a difference
Activate employees throughout the organization with access to crucial insights for strategic decision-making and long-term performance.
Rely on software you won’t outgrow
With low total cost of ownership and seamless, fee-free upgrades, this is the platform that grows with you – wherever you start and wherever you’re headed next.

Count on us at every stage
You don’t have to go it alone – we’re here to guide you along the best path. We’re EHS & sustainability experts who have been in your shoes and understand your challenges.
Tailor the platform to your needs
Infused with industry best practices, our flexible platform can be configured to fit the specific challenges and opportunities of your business.
“I can’t overstate the value of having expert knowledge and coaching from the consultants during configuration and implementation. Our previous vendor didn’t have nurses, safety professionals, or industrial hygienists on staff. They had IT people. The Cority experts have so much breadth of experience and are committed to your success.”

“The flexibility of the tool, to be able to really design what we want to do and the kind of calculations we needed to perform specifically, was a key factor in choosing Cority. The Cority software had the level of flexibility we needed to meet our requirements.”

“We use collision frequency to gauge each facility’s performance in preventing collisions and as an industry benchmark. We also use Cority to track the direct cause and types of collisions. This allows us to focus our prevention efforts on the leading types of collisions.”

“At the Ashley plant where we use the entire Cority system, we see major benefits in analysis capabilities & in cross-referencing between different areas that were previously isolated. We wanted the same benefits on a divisional level. We wanted better capability to evaluate the entire business and to implement best practices through out every facility. Today, we can look at divisional activity, view nonconformances at every plant, find the root causes, and develop permanent fixes.”

Latest News
Cority Wins Exposure Assessment & Risk Management Categories in Prestigious 2024 OH&S Industrial Hygiene Award

Recognition marks the fifth & sixth Occupational Health & Safety wins for global EHS software provider

Improving performance & reducing risk?
Enhancing total worker health & engaging employees?
Satisfying shareholders & regulatory compliance?
We’re here to help. Read On.
Featured EHS & ESG Content
Making an Impact: Looking at Sustainability through the lens of EHS
Better Together: The Power of an Integrated Health and Safety Software Solution
Navigating Sustainability Reporting: A Comprehensive Guide to ESRS, CSRD and other Reporting Requirements in the EU
[INFOGRAPH] Carbon Tracking for Private Markets Investors
Corporate Sustainability: A Journey of Progress Across the Hospitality, IT, and Healthcare Industries
Measuring Performance: Your Guide to Health and Safety Leading Indicators
5 Reasons to Embrace SaaS for EHS Software
What is the Task Force on Climate-Related Financial Disclosures (TCFD) and who does it impact?
Stay Informed!
Sign up for EHS and ESG news and best practices. Get notified on upcoming webinars, exclusive events, and industry news.